
China's self-developed 1st mRNA COVID-19 vaccine approved for emergency use
Vaccine's efficacy remains at 85.3% from 14th to 28th day after a booster vaccination, says its manufacturer firm
By Riyaz ul Khaliq
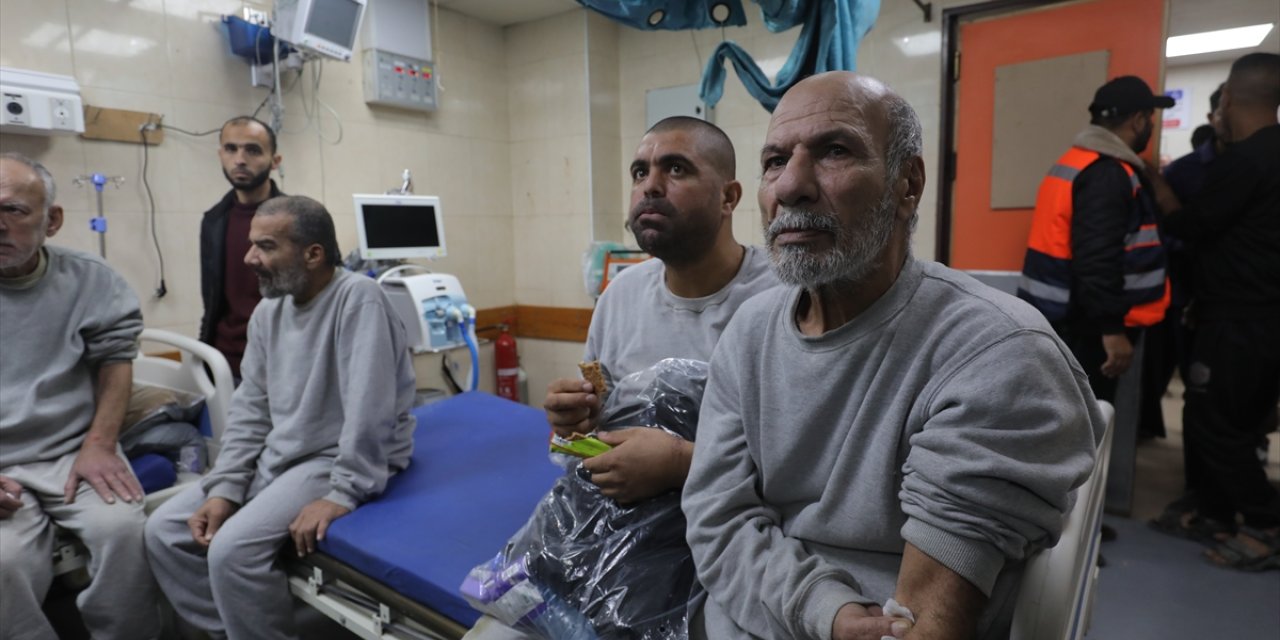
ISTANBUL (AA) - China has approved its first mRNA COVID-19 vaccine, its manufacturer CSPC Pharmaceutical Group Limited said in a filing on the Hong Kong stock exchange on Wednesday.
The mRNA vaccine, China's first self-developed product, was approved for emergency use by China's National Medical Products Administration, the Chinese daily Global Times reported.
An mRNA vaccine is a type of vaccine that produces an immune response by using a copy of a molecule known as messenger RNA.
“The efficacy of the mRNA vaccine was 70.2% from the 7th to 28th day after booster vaccination, with recombinant protein vaccine used as a control in a clinical study of 4,000 cases of sequential booster immunization conducted between December 10, 2022, and January 18, 2023,” the pharmaceutical firm said.
It added that the vaccine's efficacy "remained at 85.3% from the 14th to 28th days after a booster vaccination."
The company has been granted emergency use authorization in mainland China.
The first cases of the COVID-19 pandemic were reported in Wuhan city of central China, in Dec. 2019, and later spread across the world.
According to the World Health Organization, the COVID-19 infection has caused around 6.8 million deaths since it was first reported.
The WHO has recorded 761,071,826 confirmed cases of the disease.
Kaynak:![]()
This news has been read 192 times in total






Türkçe karakter kullanılmayan ve büyük harflerle yazılmış yorumlar onaylanmamaktadır.